The Phases of Matter
Introduction
Matter can exist in several distinct forms which we call phases. We are all familiar with solids, liquids and gases. Whether a substance is a solid, liquid or gas depends on the potential energy in the atomic forces holding the particles together and the thermal energy of the particle motions. The pressure on the subtance also has an effect on the phase.
Solids
Crystaline Solids
Crystaline solids are characterised by a long-range order. The atoms are closely packed on lattice points held in in place by atomic bonds. The internal energy of the atoms is not sufficient to allow the atoms to break away from their lattice positions. Examples of crystaline solids include semiconductors, quartz, salt, etc.
Amorphous Solids
Amorphous Solids are still closely packed together but lack the translational symmetry of crystaline solids. However, even amorphous solids have relatively good spatial ordering, especially over small distances, (10-100 molecules)
Liquids
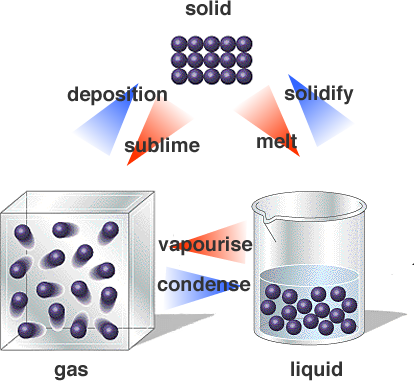
As the material is heated, the internal energy is increased and the atoms are no longer tied to their lattice positions but can move relative to each other although the atoms are still closely packed together.
Gases
A gas is matter in which the molecules are widely separated, move around freely, and move at high speeds. Examples of solids include the gases we breathe (nitrogen, oxygen, and others), the helium in balloons, and steam (water vapor).
Plasmas
Eventually, given enough heat, the electrons and nucleus become separated and into positively, charged ions and negatively charged electrons. This soup of ions and electrons is known as a plasma
Degenerate Materials
Bose-Einstein condensate is a state of matter that occurs at extremely low temperatures, near absolute zero. These temperatures are too low to occur anywhere on Earth except in laboratory experiments. The very slow motion of molecules at these temperatures allow some of the more bizarre aspects of quantum mechanics to manifest themselves in the form of novel macroscopic properties. For example, when the temperature of Helium-4 goes below 4.2 K it condenses into a liquid, at below 2.17 K, it exhibits a number of strange behaviours as a result of becoming a superfluid. In this state the viscosity disappears and it can flow with out friction. A superfluid in a beaker will form a film that crawls up the walls, over the top, and down the sides until the beaker is emptied. Another interesting effect is quantised vorticity. In a rotating container of He-4, a vortex can form in the middle, with fluid moving around in a circle, much like a water vortex around a plughole in a bath. The difference is that only certain fluid velocities are allowed. At a given distance from the vortex center, there is a minimum velocity, then twice that minimum, then three times the minimum, etc. No velocitys between these values are permitted, so the vortices are said to be quantized.
High Pressures
Change in states are caused by changing the internal energy of the material, however under extremely high pressure, ordinary matter undergoes a transition to a series of exotic states of matter collectively known as degenerate matter. These are of great interest to astrophysics, because these high-pressure conditions are believed to exist inside stars that have used up their nuclear fusion "fuel", such as white dwarves and neutron stars.
Phase Diagrams
The phases of the material can be recorded for many different pressures and temperatures. Plotting the phases, whether the material is solid, liquid or gas for many different pressures and temperatures we can build up a phase diagram for the substance. As shown in Figure 2.
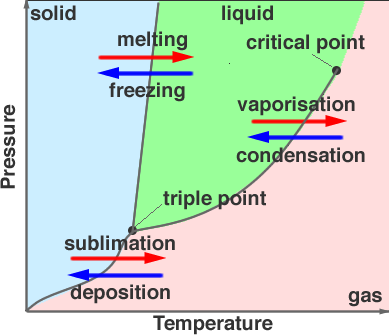
The phase diagram shows that at the interfaces between solid and liquid, liquid and gas and solid and gas it is possible for more than one phase to exist in equilibrium. The point at which all three phases come to gether is the triple point and represents the temperature and pressure for which all three states of matter can exist. For water this is, 273.16 K at 611.2 Pa. The other labeled point on the diagram is called the critical point, also called critical state. At this point the liquid and gaseous phases of a pure stable substance become identical.
